Calculate the time it takes to deplete a cylinder of gas.
Quick Links:
Recent efforts to standardize definitions between multiple codes and publications have resulted in significant confusion, particularly relating to air designated for use in CSP or the endoscopy department. Instrument Air is suitable for these applications, but as a Medical Support Gas must meet all NFPA 99 requirements and can therefore by very costly.
Class Zero Air, which may also be referred to as Equipment Air, Tool Air, or a host of other names, is a new category intended specifically to meet the need for instrument-quality air without the trappings of full compliance with the NFPA 99. Within this category, the equipment can be matched exactly to the application.
Class Zero Air, named in terms of oil content as defined by ISO 8573-1:2001 Part 1, is the perfect match for almost any non-medical compressed air application. These systems are
frequently used to deliver clean air to blow guns in CSP, cart washers, sterilizers, and scope drying cabinets such as the STERIS Reliance™ or Cantel/Medivators ENDODRY™. Class Zero Air enclosed scroll compressors have small footprints and run quiet (<60dB) so they can typically be installed either in or near the application area, even if it is staffed. These packages are not required either to have the same redundancy as medical packages or the same alarms.
Backfeeding medical gas systems can be used to provide the functionality to a zone during construction. A well-planned and documented procedure is required and recommended by the ASSE 6000 series.
This plan only applies to positive pressure gas systems. It is important to plan the shutdown in advance. Identify the responsibilities of the shutdown and backfeed. Develop an equipment list and a shutdown notification form.
SCOPE
Applies only to positive pressure gas systems.
Plan the shutdown in advance.
Identify the responsibilities during the shutdown.
After the shutdown and backfeed.
Recommended Equipment list.
Suggested shutdown notification form.
The Emergency Oxygen Supply Connection (EOSC) is used to supply oxygen to the hospital when the bulk oxygen supply is not capable of supplying the proper pressure or flow of oxygen.
As an example, a facility with a vaporizer rated for 16,000scfh for 8 hours at 70 degrees is capable of running continuously 24/7 at a rate of 2,000scfh. If the existing vaporizer is icing at 50% or more, it is running at capacity.
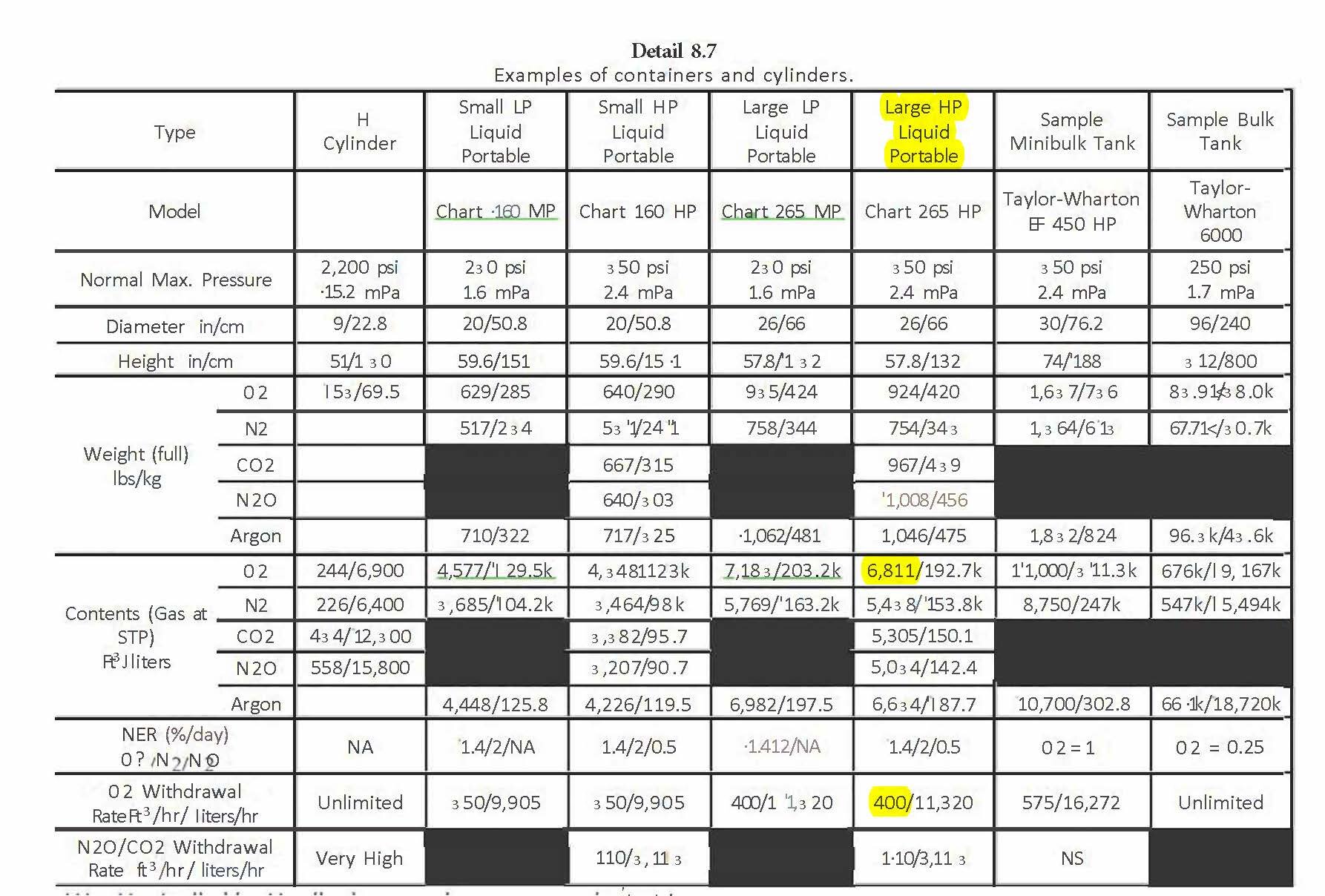
The dewar we will use in this example is the Chart Large HP Liquid Portable. It has total contents of 6,811 scf and a maximum withdrawal rate of 400 scfh with a portable evaporator or 270 scfh using the built-in internal vaporizer.
We will use the portable evaporator option with ganged dewars and dual delivery regulators.
At 400 scfh with the portable vaporizers, dewars used per hour = 2000scfh/400 scfh = 5 dewars/hour.
Detail 8.7 shows the specification and capabilities of various containers including “H” cylinders, liquid/ HP containers.
Temporary Bulk Oxygen alarms
Bulletin 114
If a temporary oxygen line is being installed to feed the hospital while work is performed:
Some of the requirements are; the temporary pipeline must be verified, the temporary bulk station must be verified, all bulk oxygen alarms signals must be present on the temporary bulk station and they must be hooked into the master alarm system, then tested and verified.
In NFPA 99 2021 a section was added to clarify the requirement of master alarms when using the EOSC.
The section is 5.1.3.5.13.2(8). Four alarm connection points installed to both master alarm panels to allow the temporary supply to be monitored while in use.
This will be a challenge. Very few installations have these signal wires available. It will require an upgrade to the EOSC. New EOSCs can be purchase with landings for these signals. Replacing the exiting EOSC is not required, A terminal strip can be added inside the EOSC box. These are low voltage connections and should be installed per the current local and national codes.
There are no exceptions for temporary systems regarding verifications or alarming on the master alarm system.
See below:Per NFPA 99 2012-2021
5.1.12* Performance Criteria and Testing — Category 1 (Gases,
Medical–Surgical Vacuum, and WAGD).
A.5.1.12
All testing should be completed before putting a new piping system, or an
addition to an existing system, into service. Test procedures and the results of all
tests should be made part of the permanent records of the facility of which the
piping system forms a part. They should show the room and area designations,
dates of the tests, and name(s) of the person(s) conducting the tests.
5.1.12.1 General.
Whenever an MGVS is installed, renovated, or has a major repair performed,
specific performance and acceptance tests are required to verify that the system
is operating within the design criteria and code requirements.
The testing measures three main areas of the MGVS: integrity of a delivery
system, quality of delivered gases, and proper operation of the MGVS
components. Because the gases and vacuum can be used for life supporting
patient care, it is critical that systems and equipment meet the performance
criteria of the code to ensure these systems are safe and reliable for patients,
staff, and visitors within the hospital.
5.1.12.1.1
Inspection and testing shall be performed on all new piped medical gas and
vacuum systems, additions, renovations, temporary installations, or repaired
systems to ensure, by a documented process and procedure, that all applicable
provisions of this document have been adhered to and system integrity has been
achieved or maintained.
5.1.12.1.2
Inspection and testing shall include all components of the system, or portions
thereof, including, but not limited to, gas bulk source(s); manifolds; compressed
air source systems (e.g., compressors, dryers, filters, regulators); source alarms
and monitoring safeguards; master alarms; pipelines; isolation valves; area alarms;
zone valves; and station inlets (vacuum) and outlets (pressure gases).
NFAP 99 2012 requires secondary equipment attached to the medical vacuum system to have a preventative maintenance program in place. Medical oxygen flowmeters are also required to be tested as a part of the biomedical preventative maintenance program.
OXYGEN ENRICHED ATMOSPHERE
NFPA 99 2018 3.3.129 Oxygen-Enriched Atmosphere (OEA). For the purposes of this code, an atmosphere in which the concentration of oxygen exceeds 23.5 percent by volume.
ACTION ITEMS:
Increase the delivery pressure from the bulk oxygen to 55 psi. Then monitor the pressure in high-risk areas. If the pressure drops to 40 psi, there will be a low pressure alarm.
If you want to know the maximum delivery flow available for an area, insert an oxygen flowmeter into every available oxygen outlet and tabulate the accumulated flow until the pressure drop for the zone drops to 45 psi.
Monitor the bulk oxygen delivery pressure and the vaporizer icing.
Develop a clear understanding with your medical gas supplier regarding the filling of the bulk oxygen, their ability to supplement the bulk oxygen supply and their delivery of oxygen tanks to RT.
When there is high usage of the medical oxygen in a hospital there will be signs of high use at the bulk oxygen station. The key point here is to monitor the ice on the evaporators and coordinate with the bulk oxygen supplier to mitigate icing.
If you know how the bulk station works, then you can create a plan to monitor it and maintain it.
Icing - Normal and Abnormal
Remedies to Icing
Daily Performance Logs
Icing - Normal and Abnormal, Normal icing can occur around the economizer located directly under the tank. It can also be normal for some vaporizers to ice due to changes in weather, environmental changes such as a new structure blocking the sun or the addition of oxygen outlets within the facility. When an increase in ice is noticed, that is the time to investigate to determine if it is problematic. Abnormal icing can occur when there is excessive demand from the facility or when there is a leak in the bulk system piping. If there is a leak, contact the bulk oxygen service company. A leak can be identified by an abnormal accumulation of ice on pipes leading to the evaporator or pipe on the vessel. If it is abnormal icing of the vaporizers there are a couple of remedies.
Remedies to Icing of the vaporizers. Vaporizers are installed in a redundant fashion. There is a switchover valve that allows you to choose which one is in service. During periods of high use it is recommended to change the IN-Use vaporizers more often. If there is no regular procedure for this, start with changing the vaporizer in-service every 12 hours. If icing continues to be a problem, change that period to every 6 hours. If one of them is over icing and alternating vaporizers does not make an improvement, use the changeover switch to put the backup vaporizer online. Then allow the iced vaporizer to thaw. If over icing becomes unmanageable using switchover, deicing can be used. Consult with your bulk oxygen service company before doing this. Power washing with heated water is a remedy. There are professional services that can do this.
Daily Performance Logs should be kept. They should include pressure readings, liquid level readings and a record of the visual inspection for ice. Any evidence of frost starting to form on the pipes exiting the vaporizer should be considered serious and remediation should start immediately. Ice buildup beyond the vaporizer, such as in the area of the switchover valve or the delivery regulators can impact the delivery of oxygen to the facility.
The operation of a bulk oxygen system involves changing the stored liquid oxygen into usable gaseous oxygen. This is a simple process because the very cold liquid oxygen wants to become gaseous just like the ambient air that surrounds its vacuum sealed container. To accelerate that process a vaporizer is used to provide more surface area to transfer ambient heat to the liquid oxygen flowing through it.
In addition, two vaporizers are provided in the event that one becomes overcome by ice that reduces the efficiency of the vaporizer.
A Switchover Valve is provided to either manually switch from one vaporizer to the other or in some cases there is an automatic switchover that switches vaporizers automatically by a timer. If there is an automatic switchover valve, it has a manual over-ride.
The diagram show the flow of liquid oxygen, in blue, and gaseous oxygen, in green. The oxygen enters the vaporizers as a liquid and leaves the vaporizer as a gas. It then goes through a regulator manifold shown here as a simple single line regulator and then to the hospital.
Problems occur when portions of the system accumulate to much ice. For most systems a certain amount of icing is normal. When an increase in ice is noticed, that is the time to investigate to determine if it is problematic. Normal icing could be around the economizer located under the tank and the vaporizers during times of high demand or cold weather.
The first line of defense in icing of a vaporizer is to use the switchover valve to move the flow over to the vaporizer that was not in use.
An area of great concern would be any frost that appears on either of the pipes that connect the vaporizers to the switchover valve.
Preventative maintenance would include daily visual inspections noting the level of icing in any areas of icing and delivery gauge readings.
Preventative deicing measures can be performed. It is recommended to always consult with the bulk system maintenance provider for any maintenance. There are professional high pressure services that provide hot to warm water high pressure to removed ice.
NEW SUPPLEMENTAL MEDICAL AIR COMPRESSOR – DESIGNED FOR COVID 19
ADD MEDICAL AIR TO YOUR EXISTING SYSTEM THE EASY WAY.
This supplemental package can be installed directly to your existing medical air system before the line regulators without brazing or a shutdown.
If your medical air compressor system is running at or near capacity, or you expect it to, you can supplement it without a shutown with a Supplemental Medical Air Compressor.
IN STOCK - This Supplemental Medical Air Compressor (SMAC) package can be installed directly to your existing medical air system, before the line regulators, without brazing or a shutdown.
This NFPA 99 compliant medical air system is designed to be used for supplementing existing medical air systems without interruption of service. These compressors are on the shelf right now.
This SMAC is designed with an enclosed, oilless, scroll air compressor- filtered to .1u and sent through a desiccant air dryer per NFPA 99- into the existing medical air plant between the existing dryer and final line regulator. This allows for easy installation without any interruption of service, and easy removal when the package is no longer needed. This option will have to be verified for use on any proposed system. The new supplemental system will be installed at a slightly higher pressure (5psi) than the existing so that it carries the load, but the existing wil still come on automatically if demand exceeds the new package capacity. If the existing package is called online, it will still have full lead/lag functionality with all required alarms.
In addition, the supplemental package meets the new requirements in the 2017 AAMI ST79, 2019 AORN Guidelines for Perioperative Practice, and 2018 FGI Guidelines for sterile processing/scope processing air. Most facilities do not currently have air that meets this quality standard for this application. The proposed supplemental package could be repurposed after the COVID-19 crisis to meet that need.
EMAIL: jim@gasmedix.com
The frequency recommendations for nitrous oxide testing is very limited. The only government document that I am aware of is the 1977 NIOSH publications which recommend quarterly. In 1977 the only practical method was badge testing so that is what they reference. Now we have on-site, real-time testing analyzers. As always, the facility policy can dictate the frequency but any frequency less than annual will meet heavy scrutiny by CMS and most state authorities.
Proper testing includes:
· Nitrous oxide testing for non-use conditions with a baseline excursion limit of 5 ppm
· Nitrous oxide testing for in use condition, live or simulated, with an excursion limit of 25 ppm
· Air exchanges should be referenced from air exchange tests or performed with the N2O test
· Positive pressure should be referenced from air positive pressure tests or performed with the N2O test
References:
NIOSH 1977 N2O Testing Frequency
CMS has issued guidance on waivers for inspection, testing and maintenance for health care facilities with a Waiver ITM Recommendations Table and a Waiver Template for Testing and Maintenance to be submitted together.
Regarding medical gas systems, we are recommending the healthcare organization issue a “state of emergency” organizational policy. This policy would defer all testing except master alarms, operating rooms, and a code review for the systems including any temporary installations or setups. Maintenance of medical gas outlets would be continued on an as-needed basis.
Medical Gas system: Testing and maintenance in section EC.02.05.09, EP 7
Dependent on HCO policy: If test was due during SOE, add 60-day grace period after lifting SOE
Air Ventilation Testing: Testing and maintenance in section EC.02.05.01, EP 15
No deferment
Anesthesia Machine: Testing and maintenance in section EC0.02.04.03. EP 2
No deferment
Downloads for Waiver Application Template and Waiver ITM Recommendations
Other CMS Blanket Waivers COVID 19
Credit: Thank you to ASHE for providing information on their web site that helped to make this article.
Medical Air Compressors Consumption Calculation p1.1
The demand on healthcare facility medical air compressors has been increased by the number of ventilators put in service. Here we look at an easy way to measure the current demand on the compressors by measuring consumption.
Calculating Air Consumption in SCFM:
This is done during the off cycle of the compressor and you record the pressure of when the system went off say (105 psig) on at (85 psig) and the (time sec) it took to go from 105 to 85. This will provide the consumption at the time of the recorded data. Consumption can vary in the system based on the demands throughout the day for OR’s and patient capacity and the use of ventilators.
Consumption (SCFM) = (.55 x G x ΔP) / t (sec)
SCFM = Standard Cubic Ft / Second
G = Gallon Size of Receiver (typically 120,200,240)
ΔP = PSIG Off to PSIG On (typically 20 psig most systems)
t (sec) = time it takes for the pressure to drop from it’s fully pumped up pressure (compressor cut out) to the when the compressors starts again ( compressor cut in)
Example:
SCFM = (.55 x 200 x 20) / 60
SCFM = 36.6